Solvent-mediated spray pyrolysis of 2D vanadium oxide nanostructures for high-performance energy storage applications
- Journal
- Electrochimica Acta
- Vol. (No.), pp.
- 498, 144628 (Sep 2024)
- Year
- 2024
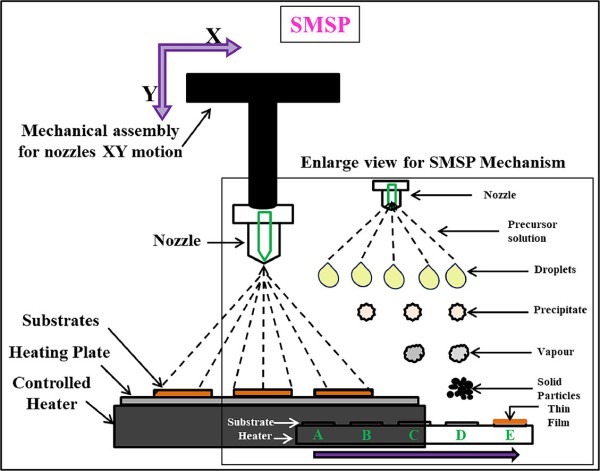
Solvent-mediated spray pyrolysis (SMSP) is recognized as a flexible method for fabricating a variety of nanomaterials, such as 2D vanadium oxide nanostructures. This process involves atomizing a precursor solution onto a hot surface, leading to the evaporation of the solvent and decay of the precursor, culminating in the development of the target nanomaterial. The inclusion of a solvent in SMSP enhances the ability to tailor the morphology and size of nanostructures and facilitates the creation of nanomaterials on diverse substrates. The current research utilized SMSP to produce 2D vanadium oxide nanostructures aimed at advanced energy storage applications. A 0.05 M solution of ammonium metavanadate (NH4VO3) in 50 ml of various solvents, including methanol, ethanol, and propanol was prepared. This solution was then sprayed onto a preheated stainless-steel substrate (673 K) using an automated spray nozzle, with compressed air serving as the carrier gas at a rate of 10 L/min. The solution flow rate was consistently held at 10 ml/min, and the optimal distance between the nozzle and substrate was determined to be 30 cm. Characterization of the resulting 2D vanadium oxide nanostructures underwent multiple physical and electrochemical techniques. The XRD results confirmed the formation of a tetragonal crystalline structure. The SEM/TEM images revealed nanostructures with a rectangular plate-like morphology with an average 13.24 nm thickness. Electrochemical evaluation was conducted by a standard three-electrode system in a 1 M Na2SO3 electrolyte, where the nanostructures synthesized with methanol solvent demonstrated a superior specific capacitance of 721.28 F/g at a 5 mV/s scan rate as compared to those synthesized with other solvents. Additionally, these nanostructures retain over 90 % of their initial capacitance after 3000 charge-discharge cycles. The remarkable specific capacitance and durability through numerous cycles highlight the potential of those 2D vanadium oxide nanostructures as promising candidates for electrodes in high-performance energy storage devices.